11.3 Absolute Ages of Rocks
11.3 Absolute Ages of Rocks
Lesson Objectives
- Describe radioactive decay.
- Explain radiometric dating.
Vocabulary
- absolute age
- carbon-14 dating
- half-life
- isotope
- radioactive decay
- radiometric dating
Introduction
The age of a rock in years is its absolute age. Absolute ages are much different from relative ages. The way of determining them is different, too. Absolute ages are determined by radiometric methods, such as carbon-14 dating. These methods depend on radioactive decay.
Radioactive Decay
Radioactive decay is the breakdown of unstable elements into stable elements. To understand this process, recall that the atoms of all elements contain the particles protons, neutrons, and electrons.
Isotopes
An element is defined by the number of protons it contains. All atoms of a given element contain the same number of protons. The number of neutrons in an element may vary. Atoms of an element with different numbers of neutrons are called isotopes.
Consider carbon as an example. Two isotopes of carbon are shown in Figure below. Compare their protons and neutrons. Both contain 6 protons. But carbon-12 has 6 neutrons and carbon-14 has 8 neutrons.
Isotopes are named for their number of protons plus neutrons. If a carbon atom had 7 neutrons, what would it be named?
Almost all carbon atoms are carbon-12. This is a stable isotope of carbon. Only a tiny percentage of carbon atoms are carbon-14. Carbon-14 is unstable. Figure below shows carbon dioxide, which forms in the atmosphere from carbon-14 and oxygen. Neutrons in cosmic rays strike nitrogen atoms in the atmosphere. The nitrogen forms carbon-14. Carbon in the atmosphere combines with oxygen to form carbon dioxide. Plants take in carbon dioxide during photosynthesis. In this way, carbon-14 enters food chains.
Carbon-14 forms in the atmosphere. It combines with oxygen and forms carbon dioxide. How does carbon-14 end up in fossils?
Decay of Unstable Isotopes
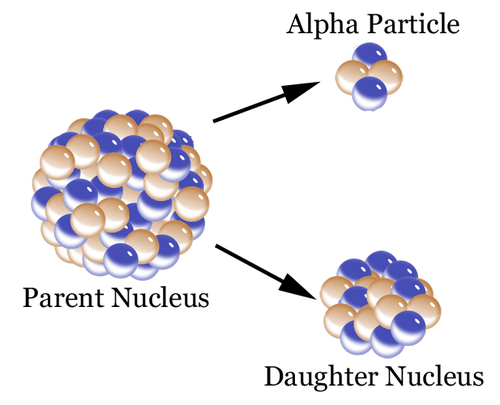
Like other unstable isotopes, carbon-14 breaks down, or decays. For carbon-14 decay, each carbon-14 atom loses an alpha particle. It changes to a stable atom of nitrogen-14. This is illustrated in Figure below.
Unstable isotopes, such as carbon-14, decay by losing atomic particles. They form different, stable elements when they decay. In this case, the daughter is nitrogen-14.
The decay of an unstable isotope to a stable element occurs at a constant rate. This rate is different for each isotope pair. The decay rate is measured in a unit called the half-life. The half-life is the time it takes for half of a given amount of an isotope to decay. For example, the half-life of carbon-14 is 5730 years. Imagine that you start out with 100 grams of carbon-14. In 5730 years, half of it decays. This leaves 50 grams of carbon-14. Over the next 5730 years, half of the remaining amount will decay. Now there are 25 grams of carbon-14. How many grams will there be in another 5730 years? Figure below graphs the rate of decay of carbon-14.
The rate of decay of carbon-14 is stable over time.
Radiometric Dating
The rate of decay of unstable isotopes can be used to estimate the absolute ages of fossils and rocks. This type of dating is called radiometric dating.
Carbon-14 Dating
The best-known method of radiometric dating is carbon-14 dating. A living thing takes in carbon-14 (along with stable carbon-12). As the carbon-14 decays, it is replaced with more carbon-14. After the organism dies, it stops taking in carbon. That includes carbon-14. The carbon-14 that is in its body continues to decay. So the organism contains less and less carbon-14 as time goes on. We can estimate the amount of carbon-14 that has decayed by measuring the amount of carbon-14 to carbon-12. We know how fast carbon-14 decays. With this information, we can tell how long ago the organism died.
Carbon-14 has a relatively short half-life. It decays quickly compared to some other unstable isotopes. So carbon-14 dating is useful for specimens younger than 50,000 years old. That’s a blink of an eye in geologic time. But radiocarbon dating is very useful for more recent events. One important use of radiocarbon is early human sites. Carbon-14 dating is also limited to the remains of once-living things. To date rocks, scientists use other radioactive isotopes.
Other Radioactive Isotopes
The isotopes in Table below are used to date igneous rocks. These isotopes have much longer half-lives than carbon-14. Because they decay more slowly, they can be used to date much older specimens. Which of these isotopes could be used to date a rock that formed half a million years ago?
| Unstable Isotope | Decays to | At a Half-Life of (years) | Dates Rocks Aged (years old) |
|---|---|---|---|
| Potassium-40 | Argon-40 | 1.3 billion | 100 thousand – 1 billion |
| Uranium-235 | Lead-207 | 700 million | 1 million – 4.5 billion |
| Uranium-238 | Lead-206 | 4.5 billion | 1 million – 4.5 billion |
Lesson Summary
- The age of a rock in years is its absolute age. The main evidence for absolute age comes from radiometric dating methods, such as carbon-14 dating. These methods depend on radioactive decay.
- Radioactive decay is the breakdown of unstable isotopes into stable elements. For example, carbon-14 is an unstable isotope of carbon that decays to the stable element nitrogen-14. The rate of decay of an isotope is measured in half-lives. A half-life is the time it takes for half a given amount of an isotope to decay.
- Radiometric dating uses the rate of decay of unstable isotopes to estimate the absolute ages of fossils and rocks. Carbon-14 can be used to date recent organic remains. Other isotopes can be used to date igneous rocks that are much older.
Lesson Review Questions
Recall
1. Define absolute age. How does it differ from relative age?
2. What is radioactive decay?
3. How do different isotopes of the same element differ? How are they the same?
4. Describe how carbon-14 forms and decays.
5. What is radiometric dating?
Apply Concepts
6. Carbon has a third isotope, named carbon-13. Apply lesson concepts to infer how many protons and neutrons are found in each atom of carbon-13. Carbon-13 is a stable isotope, like carbon-12. How useful would carbon-13 be for radiometric dating?
Think Critically
7. Explain how carbon-14 dating works.
8. Compare and contrast carbon-14 dating and potassium-40 dating.
Points to Consider
Scientists estimate the ages of rock layers in order to better understand Earth’s history and the history of life.
- What do you already know about Earth’s history? For example, do you know how Earth formed?
- How old is Earth? When did the planet first form? And when did life first appear?
Lesson 11.2, Lesson Review Question 6 image: Image copyright branislavpudar, 2014. http://www.shutterstock.com. Used under license from Shutterstock.com.
- Log in or register to post comments
- Email this page